Course:ECON371/UBCO2010WT1/GROUP2/Article6
Back to
Group 2: The Environmental Impacts of Natural Gas Extraction
Article 6: Natural Gas Drilling Produces Radioactive Wastewater
Summary
The New York State’s Department of Environmental Conservation took wastewater samples and found out that the water from natural gas drilling contains radioactive radium 226. The radium levels were thousands of times higher than the safety limit for people's use and consumption and 267 times over the discharge limit. Human exposure to radium can cause health problems, such as cancer and other disorders. Despite that, the health department stated that the water contains very low radiation levels, and it will not affect people exposed to it. Given the fact that the state officials found such high concentrations of a radioactive element, the government is likely to tighten the policy for discharging waste water.
Analysis
The article states that concentrations of the radioactive element radium 226 were found to be very high in the waste water resulting from natural gas drilling; however, the radiation levels are low. This statement may sound paradoxical, unless the half-life of radium 226 is considered. This half-life is 1601 years, meaning that in a human life-time, a very small fraction of a given radium sample will undergo radioactive decay. As such, it is reasonable to say that even if people are exposed to high levels of radium 226, they are not exposed to high levels of radiation. If this fact is considered, the damages assumed to be caused by radium decrease significantly. However, a thorough scientific examination of the health impacts of radium is required to find the true marginal damage curve for the emissions of this element.
Assuming that the article's author has a good idea of what a safe level of radium is, the fact that the concentration found in waste water is thousands times this level is troubling. The article suggests a need for radioactive licensing. This may imply a permit system or a standard system. If a permit system is used to control emission levels, then polluters would be able to trade the right to pollute. In the case of a radioactive material in water, this policy may be highly criticized by the public for giving the wealthy companies the right to seriously harm people's health with radioactive emissions. Thus, a standard system may be more welcome. Here, the option would be between an individualized or a universal standard. In the case of an individualized standard, the efficient point is more likely to be reached. However, this policy may end up being expensive in terms of information required to establish and enforce the standard level when many polluting wells are being regulated. Some of these wells may be in close vicinity to a city's water supply and thus cause higher marginal damages than more distant wells. As a result, not only the marginal abatement cost curve, but also the marginal damage curve will differ among polluters. This will make it hard to determine an intersection of the two, known as the efficient point. Thus, it may be cheaper to set a universal standard which will apply to all wells regardless of size, level of production, or distance from a city. This policy will not be cost-effective since the equimarginal principle will not be satisfied as the MAC curves of various wells are likely to be different. However, the policy may achieve a desired level of emissions and will also create a high incentive for innovation for firms with steep MAC curves.
With all of the policies suggested above, there is an incentive for the polluters to overstate their MAC curve in an attempt to either get an excess of permits to sell or to get a loose standard. The advantage of the tradeable permit system lies in the ability of regulator to monitor the permit market. If firms have overstated their MAC curve, an excess of permits will be available, and their prices will be lower than expected. From this, the regulator can derive the true efficient point and thus adjust the number of available permits. With a standard system, this is not possible. Polluters will either meet the standard or not. If the standard is too loose, total damages will exceed total abatement costs. In the case of a highly dangerous radioactive substance, which we will assume radium to be, this situation is very undesirable. The cost of being wrong is very high. Thus it may be needed to have a tradeable permit system in place, despite public critique, until the true efficient point is determined.
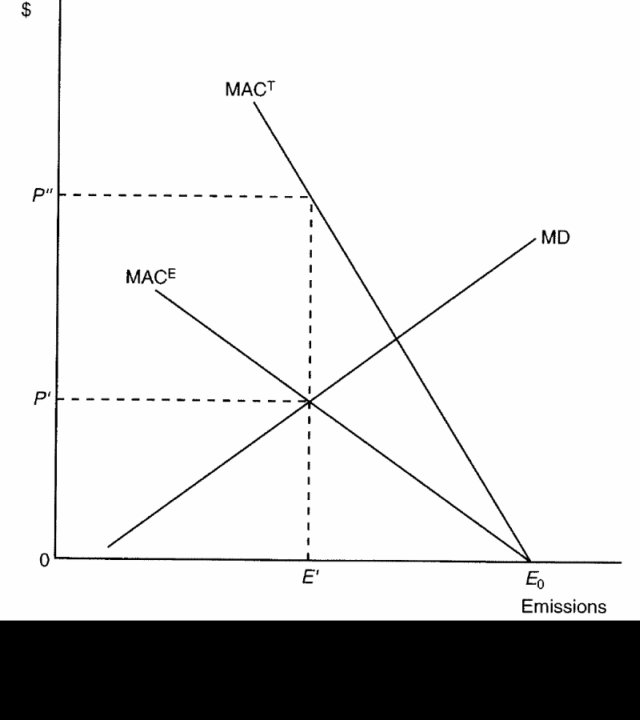
Through a transferable discharge permit (TDP) system, a firm's true MAC will be revealed when it purchases permits at the price of P" rather than at P'. [See graph below]
[Image borrowed from Barry Field and Nancy Olewiler's Environmental Economics (Updated 2nd Cdn. ed.), p.271.]
Prof's Comments
In this particular situation, we are moving into the realm of toxics. I agree to a degree with your comments about radioactivity. However, that does not address the question of the safety of the mineral Radium itself. Also, public perception is very important, and the public is generally very frightened of anything radioactive. Built a cloud chamber myself as a grade 10 high school science project and had bits of uranium ore for my radiation source, all of which was pretty safe. However, with perceptions, the MD curve people are acting on may be very high, with the result that it is 'efficient' to have very low levels of radium.