Course:FNH200/Lessons/Lesson 04
Food Standards, Regulations and Guides - Food Additives
04.0 Overview
This lesson will introduce you to the concept of food regulation and you will become familiar with "standards of identity", standards and quality grades for foods, as well as the major governmental agencies that have a role in regulating the safety and quality of the food supply. You will learn about the Food and Drugs Act and Regulations of Canada, and the regulations that govern labelling and advertising as they apply to food. We will also discuss regulations governing grade standards for various food commodities that are administered by the Canadian Food Inspection Agency.
At one time or another, most people have been in a conversation where food additives have been the topic of discussion. Few people know what food additives are, what their purpose is, and what regulations govern their use. In this lesson we discuss and compare the Canadian and United States definitions of a food additive. We also discuss the classes of food additives and their functions in food systems. We review Canadian labelling requirements as they pertain to food additives and discuss the concepts employed in the evaluation of safety of food additives. We end the lesson with a discussion of aspartame and nitrites as examples of food additives that have generated much controversy in the recent past.
Objectives
After completing this lesson, you will be able to:
- discuss how regulations are established, to ensure the quality and safety of the Canadian food supply
- identify which governmental agencies are responsible for regulating the safety and quality of the food supply
- define what a food additive is
- interpret the function of food additives that are listed on the labels of ingredients of food you consume
- explain the basis upon which safety of food additives is determined; and
- articulate your set of values as they pertain to the use of food additives in foods
- compare and contrast the definition of a food additive in Canada and United States
- demonstrate the ability to do research and extract information about the Canadian food acts and regulations
Optional Readings
- Hotchkiss, J.H. and Cassens, R.G. 1987 [April]. Nitrate, nitrite and nitroso compounds in foods (A scientific status summary). Food Technology, 41(4):127-136.
- Kroger, M, Meister, K. and Kava, R. 2006. Low-calorie sweeteners and other sugar substitutes: A review of the safety issues. Please see this link INSTEAD; Comprehensive Reviews in Food Science and Food Safety 5: (read only pp. 37-39). NOTE: this is the same article as required in lesson 3.
Resources
- Health Canada, Food program: Safety of Aspartame. https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/food-additives/sugar-substitutes/aspartame-artificial-sweeteners.html
- Health Canada. 2006. Food Additive Dictionary. Publication H49-10/1996E. Ottawa: Health Canada. https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/food-additives/dictionary.html
04.1 Food Standards, Regulations and Guides
Terms to remember |
|
In order to ensure that the food we purchase and consume is safe and within certain preset limits of quality, there are government standards, regulations and grades in place to protect the consumer.
The Department of Justice Canada is responsible for maintaining the Consolidated Statutes and Regulations for the Government of Canada, including the Food and Drugs Act and Food and Drug Regulations.
The Food and Drugs Act of Canada is administered by the Health Products and Food Branch of Health Canada, whereas inspections for compliance are enforced by the Canadian Food Inspection Agency. Several other government agencies work together to ensure the safety and quality of foods produced and/or consumed in Canada.
The government agencies and their regulatory functions are listed below.
Table 4.1 Government agencies and their regulatory functions
Agency |
Regulatory Function |
| Health Canada
(Health Products & Food Branch) https://www.canada.ca/en/health-canada/services/food-nutrition.html |
|
| Canadian Food Inspection Agency (CFIA) |
|
| Measurement Canada | Agency of Innovation, Science and Economic Development Canada.
responsible for ensuring accuracy in the selling of measured goods, developing and enforcing the laws related to measurement accuracy, approving and inspecting measuring devices and investigating complaints of suspected inaccurate measurement. |
| British Columbia Ministry of Health | public health inspection of retail stores and food service establishments
inspection of provincially inspected meat processing plants and dairy processing plants |
| Municipal | public health inspection of retail stores and food service establishments |
The Food and Drugs Act
Sections 3, 4, 5, and 7 of the Food and Drugs Act form the foundation of the consumer protection laws. Excerpts of the Act are shown in Box 4.1 to give you an idea of the nature of the regulations.
It is interesting to note that Section 3 of the Food and Drugs Act prohibits the advertising to the general public of any food, drug, cosmetic or device for the treatment, prevention or cure of any of the diseases listed on Schedule A of the Food and Drugs Act. This section of the Act also prohibits the sale of a food, drug, cosmetic or device that is labeled in this manner.
In the light of recent trends and the demand for natural health products, the House of Commons Standing Committee on Health mandated an External Working Group which is overseeing the revisions may be needed to this section of the Act.
Excerpts from The Food and Drugs Act of Canadahttp://laws-lois.justice.gc.ca/eng/acts/F%2D27/page-1.html#docCont | |
Section 3(1) no person shall advertise any food, drug, cosmetic or device to the general public as a treatment, preventative or cure for any of the diseases, disorders or abnormal physical states referred to in Schedule A. (2) No person shall sell any food, drug, cosmetic or device (a)that is represented by label, or: (b) that is represented to the general public as a treatment, preventative or cure for any of the diseases, disorder or abnormal physical states referred to in Schedule A. Some of the diseases mentioned in schedule A include alcoholism, appendicitis, arthritis, cancer, depression, diabetes, heart disease, hypertension, liver diseases, obesity, sexual impotence, tumours, venereal disease. |
Section 5(1) No person shall label, package, treat, process, sell or advertise any food in manner that is false, misleading or deceptive or is likely to create an erroneous impression regarding its character, value, quantity, composition, merit or safety; (2) An article of food that is not labelled or packaged as required by the regulations, or is labelled or packaged contrary to the regulations, shall be deemed to be labelled or packaged contrary to subsection (1). |
Section 4No person shall sell an article of food that: (a) has in or upon it any poisonous or harmful substance; (b) is unfit for human consumption; (c) consists in whole or in part of any filthy, putrid, disgusting, rotten decomposed or diseased animal or vegetable substance; (d) is adulterated; (e) was manufactured, prepared, preserved, packaged or stored under unsanitary conditions. |
Section 7(a) No person shall manufacture, prepare, preserve, package or store for sale any food under unsanitary conditions. |
Box 4.1 Sections 3, 4, 5 and 7 of The Food and Drugs Act of Canada, that are the foundation of consumer protection laws.
04.2 How are Regulations established?
- The Food protection laws, in Canada, are wide in scope and major changes in the regulations embodied within the Food and Drugs Act are made after extensive consultation. We will discuss this consultation process when we deal with the section on Food Irradiation.
- Rapid changes can also be made to the Food and Drugs Act such as in the case of delisting a food additive or other substance permitted in food when new evidence arises concerning issues of safety of a particular substance. Such was the case when the non-caloric sweeteners cyclamate and saccharin were banned.
- The regulations can be amended by authority of the Governor in Council.
As noted on Table 4.1, regulations about labelling, advertising and claims about food are administered by the Canadian Food Inspection Agency (CFIA):
- CFIA deals with food labelling, advertising and claims about food.
- Administers the labelling, packaging and advertising regulations under the Consumer Packaging and Labelling Act and Regulations and the Food and Drugs Act and Regulations.
- The CFIA also reviews all advertisements, on Canadian radio and television, making claims about foods.
Weights and measures are regulated by "Innovation, Science and Development Canada" specifically by an agency known as "Measurement Canada". This agency is responsible for inspection of measurement devices and providing the accuracy certification stickers
https://www.ic.gc.ca/eic/site/mc-mc.nsf/eng/lm04710.html
Please note that packaged retail products are subjected to Consumer Packaging and Labelling Act and Canadian Food Inspection Agency ( CFIA) is responsible for overseeing their measurement accuracy.
04. 3 Food Labelling Requirements
Labelling information required on pre-packaged food products, from domestic food processors or imported products, is based on the Consumer Packaging and Labelling Act and Regulations and can be found in theat the CFIA website: http://www.inspection.gc.ca/food/labelling/food-labelling-for-industry/eng/1383607266489/1383607344939
Summarizing the core labelling requirement, a label should include the following:
- Bilingual labelling- All mandatory information on food labels must be shown in both official languages, i.e., French and English.
- Common name of the food. The common name is the name prescribed in the Food and Drugs Regulations. In the absence of a prescribed name, the name by which the food is commonly known is used.
- Country of Origin- Declaring of Country of Origin is required for some specific food. Some companies may choose to voluntarily name the country of origin as advertising. For a complete list of foods requiring mandatory declaration please see the link below
- http://www.inspection.gc.ca/food/labelling/food-labelling-for-industry/label/country-of-origin/eng/1334599362133/1334601061354
- Date marking and storage instructions as required. This is required for foods with a storage life of 90 days or less.
- Durable life is the period of time, beginning on the day on which the pre-packaged product is packaged for retail sale, during which a product stored under prescribed conditions will retain, without appreciable deterioration, its normal wholesomeness, palatability and nutritional value and any other qualities claimed for it by the manufacturer. Products that have passed the durable life date and that have been stored under prescribed conditions are still safe to eat but the quality (appearance, flavour, nutritional value) may have deteriorated.
- Identity and Principal Place of Business
- Irradiated foods
- Legibility and location
- List of ingredients in descending order of proportion
- The Nutrition Facts table will show the Calories, the amount of fat, saturated and trans fats, cholesterol, sodium, carbohydrate, fiber, sugars, protein, calcium, iron and Vitamins A and C in a specified amount of food.Nutrition facts regulations apply to all pre-packaged foods with some exemptions (e.g fresh fruit and vegetables, raw single ingredient meat and poultry that are not ground, raw fish or seafood, alcoholic beverages).
- Net quantity of the food
- Sweeteners
- Other mandatory information may be required for certain foods: eg. % alcohol for alcoholic beverages, % milk fat for some dairy products
Nutrient content claims and diet-related health claims, if made, must adhere to the stated criteria (see below)
Currently, there are 5 disease reduction claims allowed in Canada:
- Disease Risk Reduction Claims with Respect to Sodium and Potassium
- Disease Risk Reduction Claims with Respect to Calcium and Vitamin D
- Disease Risk Reduction Claims with Respect to Saturated and Trans Fats
- Disease Risk Reduction Claims with Respect to Cancer Risk Reduction
- Disease Risk Reduction Claims with Respect to Dental Caries
| Want to learn more? |
|
| Want to learn more? |
|
04.4 Standards of Food Identity and Composition
The Food and Drug Regulations contain descriptions of certain foods that specify, for example, what is allowed in those foods as ingredients. These descriptions are standards of identity and composition that have to be met for a food to be legally called by the name in the standard. The foods are referred to as "standardized foods". Examples of standardized foods include bread, milk, cheese, orange juice, sausage, jam, wine, beer, vinegar and salt. Foods that do not have a standard of identity are referred to as "unstandardized foods". Snack foods like potato chips, various bakery items such as rolls, donuts and cakes, yogurt, and pizza are examples of unstandardized foods.
Standards of food identity and composition are defined in the Food Regulations of the Food and Drugs Act of Canada
- Please bookmark the link for the Food and Drug Regulations of The Food and Drugs Act of Canada:
- http://laws-lois.justice.gc.ca/eng/regulations/C.R.C.,_c._870/index.html
Note the following:
- An identity standard is one that states what the food shall be and defines a food or ingredient. Compositional standards list the mandatory and permitted ingredients in foods.
- There are standards of identity or composition for over 300 foods in the Food Regulations in Canada. They are classified within 28 divisions. For example, Division 13 regulates "Grain and Bakery products". In this division, the standards of identity and composition for white wheat flour and bread can be found. A fragment of this division is shown below in Box 4.2 (click on the Box to read its contents).
- The dates on the left hand side of the identity and compositional standards (Box 4.2) indicate the date of the last revision of a particular section of the standard.
| Want to learn more? |
|

04.5 Food Grades Standards
Food grades standards are administered by the CFIA.
Here is a list of some of the regulations:
- Volume 1, Ovine carcasses and poultry carcasses
- Volume 2, Fresh fruit or vegetables
- Volume 3, Processed fruit or vegetable products
- Volume 4, Dairy products
- Volume 5, Eggs
- Volume 6, Honey
- Volume 7, Maple syrup
- Volume 8, Fish
| Critical Thinking |
| To complete the following activities visit the http://inspection.gc.ca/food/requirements/labelling/industry/grades/eng/1468508117774/1468508381597.
1) Find out more about regulations governing fresh fruits or vegetables.
Response: Grade standard descriptions for processed fruits or vegetables indicate that the grades are based on aesthetic qualities of the fruits and vegetables. Although there is not a great deal of information available, it appears that Canada Fancy and Canada Standard processed fruits and vegetables are similar in nutrient value. Thus the grades do not necessarily indicate that a better grade (fancy) is superior to a lower grade (standard) from a nutrient point of view. 2) Find out more about eggs. Select "Egg Regulations" and scan through the information to answer the following questions:
Response: Eggs are graded only if certain criteria are met, and then only by an inspector at a registered egg station. The eggs are evaluated for weight, cleanliness, soundness and shape of shell, shape and position of yolk in the egg during candling, size of air cell (small = fresh), abnormalities (e.g., blood spots).
3) Now select "Ovine and Poultry Carcass Grading Regulations" and browse through to answer the following
4) Now select "Maple Product Regulations" and browse through the Maple Syrup Grade Names.
|
Video
At this point you should watch the video on Egg processing, including the grading system and processing.
Can you answer the following:
- Why are the eggs washed?
- How is the process of candling done?
- What are the main external and internal characteristics that are evaluated during candling?
- Which grade(s) of eggs are normally found in the retail market? what happens to other grades? are all eggs suitable for food use?
- What types of processed egg products are shown? what are the conditions for HTST pasteurization of liquid whole and yolk?
04.6 Natural Health Products
To deal with the uniqueness of emerging products which are neither strictly foods nor drugs, the government has established a Natural Health Products Directorate, responsible for regulations and labelling guidelines for these products. We will learn about this topic in Lesson 13.
How about the international scene?
The Codex Alimentarius Commission was established in 1963 by the World Health Organization and the Food and Agriculture Organization of the United Nations to develop international food standards to protect consumer health and to facilitate fair trading practices in foods. Today, there are more than 189 member countries including Canada. Canada's participation in Codex is coordinated through the Office of the Codex Contact Point for Canada, located in the Food Directorate, Health Products and Food Branch of Health Canada.
If you are interested in the regulations in the United States, you may wish to check out the website of the US Food and Drug Administration, Centre for Food Safety and Applied Nutrition: https://www.fda.gov/
The Agriculture and Agri-Food Canada website for information concerning US FDA regulations is also useful especially for import-export cases: http://www.agr.gc.ca/eng/home/?id=1395690825741
04.7 Food Additives
There is probably no component of the food system that has generated so much discussion among the consuming public as food additives. Many myths and half-truths abound about food additives, their uses and the perceived dangers related to the presence of additives in foods in the Canadian food supply. Compounding this, is the prevalence of American radio, television, newspapers and magazines in Canada with articles about the positive and, in the majority of cases, negative aspects about food additives.
- As you will note shortly, the Canadian definition of a food additive is not the same as the definition of a food additive in the United States. This has led to much of the confusion in the eyes of the Canadian public, who at times may know more about the United States regulations and legislation than the Canadian regulations and legislation and the Food and Drugs Act of Canada.
Canadian Food additive definition
A food additive is any substance, the use of which results, or may reasonably be expected to result in it or its by-products becoming a part of or affecting the characteristics of a food.
Under the Canadian definition, the following are considered NOT to be additives:
- any nutritive material that is used, recognized or commonly sold as an article or ingredient of food
- amino acids, mineral nutrients and vitamins
- spices, seasonings, flavouring preparations, essential oils, oleoresins and natural extractives
- food packaging materials and components thereof
- drugs recommended for administration to animals that may be consumed as food.
The exceptions are not included in the definition of a food additive since regulations in other divisions of the food regulations of The Food and Drugs Act of Canada govern their use.
Now compare the Canadian definition of a food additive with the definition adhered to in the United States by the Food and Drug Administration, the federal counterpart to the Health Products and Food Branch of Health Canada.
The definition of a food additive in the United States is as follows:
"In its broadest sense, a food additive is any substance added to food. Legally, the term refers to 'any substance the intended use which results or may reasonably be expected to result-directly or indirectly-in its becoming a component or otherwise affecting the characteristics of any food. This definition includes any substance used in the production, processing, treatment, packaging, transportation or storage of food. If a substance is added to a food for a specific purpose in that food, it is referred to as a direct additive. For example, the low-calorie sweetener aspartame, which is used in beverages, puddings, yogurt, chewing gum and other foods, is considered a direct additive. Many direct additives are identified on the ingredient label of foods. Indirect food additives are those that become part of the food in trace amounts due to its packaging, storage or other handling. For instance, minute amounts of packaging substances may find their way into foods during storage. Food packaging manufacturers must prove to the U.S. Food and Drug Administration (FDA) that all materials coming in contact with food are safe, before they are permitted for use in such a manner."
Quoted from the answer to "What is a food additive?" in the International Food Information Council (IFIC) Foundation US Food and Drug Administration (FDA) Brochure: April 2010 from the http://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/ucm094249.pdf
Although the Canadian and American definitions of food additives sound somewhat similar there are substantial differences between them as illustrated in the following table. You can clearly deduce that confusion can exist among consumers getting their information from the media from two neighbouring countries:
| Considered Food Additives | Canada | United States |
|---|---|---|
| Nutritive materials, vitamins, minerals and amino acids | No | Yes |
| Spices, seasonings and flavourings | No | Yes |
| Agricultural chemical residues | Noa | Yesb |
| Food packaging components | Noa | Yesb |
| Drugs recommended for therapeutic use and as feed additives for administration to food producing animals | Noa | Yesb |
| Number of additives permitted ("on the books") | ~400 | >3000 |
a considered as contaminants in Canada.
b considered as unintentional food additives in the United States.
Justified Uses for Food Additives
The Food and Agriculture Organization (FAO) of the United Nations has stated that the use of food additives is justified when one or more of the following conditions are met:
- additives used to maintain nutritional quality of the food. Use of additives that prevent or inhibit destruction of nutrients during processing and storage (e.g., use of antioxidants to prevent destruction of linoleic acid in oils);
- additives that function to enhance the keeping quality or stability of the food with a concomitant decrease in food wastage (e.g., use of antioxidants to delay fat oxidation; antimicrobial agents to delay microbial spoilage of food);
- additives used to make foods attractive without deception (e.g., use of orange/yellow colours in margarine to provide a pleasing appearance; colouring agents are not permitted for use in fresh meats such as ground beef because a colouring agent could disguise the colour changes that signify the onset of spoilage of the meat);
- additives used to provide essential aids to food processing (e.g., use of emulsifying agents to promote formation of stable emulsions).
Food additive regulations in Canada
How are Food Additives regulated in Canada?
Look under Division 16 FOOD ADDITIVES of The Food and Drug Regulations (FDR) for a detailed list of food additives set out in tabular form: https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/food-additives/lists-permitted.html
https://laws-lois.justice.gc.ca/eng/regulations/C.R.C.,_c._870/index.html
As you will notice, the list provides the following information:
- The purpose of the food additives are listed (eg. anti-caking agents);
- The name of the additives that can be used for that purpose;
- Foods in which they are permitted and the maximum amount permitted.
Since the listing of food additives is a positive list, if a food is not listed in the tables the additive in question cannot legally be used in that food item. An example of a page from Division 16 is shown in Figure 4.2.

What is "Good manufacturing practice"?
When the maximum level of use for a food additive indicates "Good manufacturing practice" (GMP); it basically means the minimum amount of an additive required to accomplish the specific purpose for which the additive is listed.
This minimum amount is based on technical food processing needs.
There are 15 categories of food additives in Canada. The categories and examples of the additives are shown in Table 4.3 of this lesson.
Some additives are listed in more than one category since an additive can have several functions in foods. Ascorbic acid, for example, functions as a dough conditioning agent when used in bread formulations, but it is also listed as a preservative since it also has antioxidant functionality.
| Additive Category | Function |
|---|---|
| Anticaking agents |
|
| Bleaching, maturing, and dough conditioning agents |
|
| Colouring agents |
|
| Emulsifying, gelling, stabilizing and thickening agents |
|
| Food enzymes |
|
| Firming agents |
|
| Glazing and polishing agents |
|
| Miscellaneous agents |
|
| Sweeteners |
|
| pH adjusting agents, acid reacting materials and water correcting agents |
|
| Preservatives |
|
| Sequestering agents |
|
| Starch modifying agents |
|
| Food additives used as yeast foods |
|
| Carrier or extraction solvents |
|
The Food Additive Approval Process
The information that companies must provide when submitting applications to the Health Products and Food Branch of Health Canada for approval of a new food additive is listed below:
- Composition, properties, method of manufacture and specifications of the substance to be used as a food additive;
- Amount and purpose of use;
- An acceptable method of analysis to determine the presence and concentration of the proposed food additive
- Data establishing that the proposed food additive will have the intended physical or other technical effect;
- Detailed reporting of tests conducted to establish the safety of the proposed food additive. Those studies must include:
- biochemical and physiological tests;
- subacute and chronic toxicity tests; and
- reproduction studies
- A proposed maximum limit for residues of the food additive in or upon the finished food;
- Specimens of the labelling proposed for the food additive; and
- A sample of the food additive.
Reference: FDR, Division 16, B.16.002.
Note that the information required relates to both the technological properties of the proposed additive as well as the long term safety of the additive.
This type of documentation was required when the G.D. Searle Company applied to have the low caloric sweetener, aspartame, approved for use as a food additive in Canada.
When the Health Products & Food Branch obtained the information they evaluated it relative to the safety of aspartame as well as its technological properties and proposed uses in foods.
The use level permitted in specified foods was determined taking the following parameters into consideration:
- No effect level = the highest level of the chemical which caused no harmful effects in the test animals.
- No effect level for humans = no effect level in animals, divided by a safety factor. For most food additives the safety factor is generally 100.
- Acceptable daily intake = daily dosage of a chemical which during an entire lifetime appears to be without appreciable risk on the basis of all facts known at that time. The acceptable daily intake is expressed as mg intake per kg body weight.
- Without appreciable risk = the practical certainty that injury will not result even after a lifetime of exposure.
- The probable daily intake of a food additive is determined to ensure that this value would not exceed the acceptable daily intake. Food consumption estimates of particular food commodities are used to determine the probable daily intake of the food additive in question. Data from food consumption surveys as well as information from Statistics Canada and the published scientific literature are used to estimate consumption of particular food items by various groups in Canada (e.g., children, teenagers, the elderly, etc.). If the probable daily intake of the food additive in question were to exceed the acceptable daily intake, the additive would not be approved for use or it would be approved for very restricted use.
The dose-response curve below depicts the above-mentioned concepts. These parameters will be discussed again in Lesson 12.
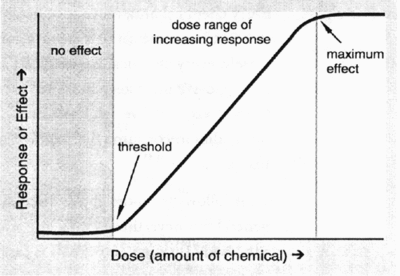
Examples of Food Additive Approval Process
Two additives, aspartame and nitrites, will now be described in order to give you some insight into the controversies which surrounded those additives and also into the decision-making processes with regard to risk/benefit issues.
| Want to learn more? |
|
Example 1: Aspartame
Aspartame is a low-calorie sweetener yielding 4 Cal/g when metabolized. On a weight basis, aspartame yields the same caloric value as an equivalent weight of sucrose. Since aspartame is intensely sweet, it can be used in very small quantities and thus can be added to sweeten "low-calorie" foods (review Lesson 3).
Aspartame was approved for use in Canada in 1981. Since its introduction as an approved sweetener, aspartame has received much attention in the media with respect to the alleged risks related to the presence of aspartame in foods.
You will note in the article that aspartame is digested in the human body to its constituent components (aspartic acid, phenylalanine and methanol) which are metabolized by normal metabolic routes. The safety aspects of aspartic acid, phenylalanine, and methanol are discussed in the article. Aspartame also has not demonstrated carcinogenicity in animal studies. Aspartame in foods can undergo degradation to diketopiperazine (DKP) during long-term storage and when it is exposed to high temperatures for extended periods of time. Studies indicate that DKP does not appear to cause any deleterious effects when ingested.
| Critical Thinking |
|
- If you consume aspartame-sweetened foods you may find it interesting to calculate your daily intake of aspartame.
- The information you would require is your weight in kilograms, the quantity of each aspartame-containing food consumed daily, as well as the concentration of aspartame in each food item (in mg aspartame/ 100 ml or 100 g, as stated on the list of ingredients for each food item).
- Note that most "diet" soft drinks in Canada now contain a blend of aspartame with Acesulfame-K.
- The risks, to metabolically normal individuals, relating to consumption of aspartame are very small while the benefits relating to use of aspartame are high, particularly for individuals wishing to decrease their caloric intake while still enjoying sweet tasting foods.
- The benefits of aspartame to diabetics are obvious. However, there is a small segment of the population for which aspartame in foods poses a substantial risk. Those individuals suffer from phenylketonuria
Consequently, according to the Canadian labelling regulations, foods to which aspartame is added must
- contain a statement on the label saying "contains Aspartame" either individually or conjunction with other sweeteners;
- list aspartame in the list of ingredients; and
- must also indicate the aspartame content expressed in milligrams per serving of the stated size.
- stating " Aspartame contains phenylalanine
This information is placed on the label of aspartame containing foods to warn phenylketonurics that they should avoid the product or consume it in very limited quantities because of their impaired ability to metabolize phenylalanine. Please read the information on PKU and aspartame in the reading (Kroger, M, Meister, K. and Kava, R. 2006).
Example 2: Nitrites
The other food additive that we will review is nitrite.
| Want to learn more? |
|
Why use nitrites?
The reactions of myoglobin, the red pigment in meat, with nitric oxide (formed from nitrites) in cured meats leads to the formation of nitrosohemochrome, the pink colour typical of cured meat products.
As shown in Figure 4.5 below, nitrite has several functions in cured meats. By far, the most important role of nitrite is to act as an antimicrobial agent, particularly towards Clostridium botulinum which produces the toxin responsible for botulism (you can find out more about Clostridium botulinum in Lesson 6).
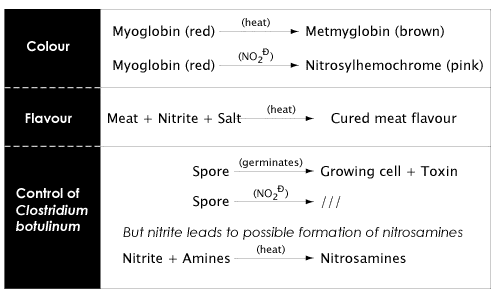
- The fact that the exact mechanism by which nitrites inhibit growth and toxin production by Clostridium botulinum are not fully understood makes the search for an alternative very difficult.
- To this date an acceptable alternative to nitrite as an antimicrobial agent in cured meats has not been found even though many years and millions of dollars, in numerous countries, have been spent in the search for an alternative for nitrite and also to gain an understanding of the mechanism(s) by which nitrite functions as an anti-botulinal agent.
What are some of the risks associated with nitrites?
There is a possibility that nitrites (naturally occurring, or added as an additive, or produced by reduction of nitrates), can react with amines to produce nitrosamines - some of which are potent carcinogens. The discovery in the 1960s of nitrosamines in foods, especially in cured meats, led to many studies and reviews on the risk/benefit situation relating to the use of nitrite and nitrate as food additives, particularly in cured meats. Much research has been conducted on the nitrosation reactions that can occur in foods as well as factors in foods that lead to nitrosamine formation.
Data indicate that foods are not a major source of nitrosamine exposure in humans, and that the greatest exposure comes from use of tobacco products. In foods, beer and fried bacon contribute more nitrosamine to the diet than all other foods combined (see the scientific status summary by Hotchkiss and Cassens). Nevertheless, whenever possible, the exposure to nitrosamines should be minimized.
In 1972, levels of nitrosopyrrolidine in excess of 100 parts per billion (ppb) were detected in fried bacon. [Oneppb is an extremely small quantity; if you were to travel one foot on a trip to the Moon, it would represent one part in one billion, since the distance to the Moon is about one billion feet.] By 1982, the level of N-nitrosopyrrolidine in fried bacon produced in the United States was in the range of 10 ppb, about ten-fold lower than the level in 1972. Bacon in the raw stage, has been found to be generally free of nitrosamines which develop during high-heat frying.
You might ask why this dramatic decrease occurred. Research into meat curing operations demonstrated several instances where nitrosation reactions were favoured during the production of cured meats. Changes in the curing process led to decreases in nitrosamine formation. In addition, it was found that compounds such as ascorbic acid, sodium erythorbate (isoascorbate) and alpha-tocopherol (vitamin E) would interfere with the nitrosation reactions. The next time you have an opportunity to read a label on a package of cured meat you will notice that ascorbic acid or sodium erythorbate are listed as one of the ingredients.
- Regulatory agencies have been faced with a dilemma as far as nitrite in foods, particularly cured meats, is concerned.
- It is known that under certain circumstances, particularly during frying of bacon, that nitrosamines can be formed.
- Some nitrosamines are potent carcinogens while others are non-carcinogenic.
- The type and quantity of nitrosamines that are formed depend on the reactants and the conditions present in the food.
The article "Nitrates, nitrites, and nitroso compounds in foods" reviews the conditions that favour as well as those that impede nitrosamine formation. It also presents the risk/benefit situation with regard to nitrates (NO-3) and nitrites (NO-2) in the diet.
What are some important facts in assessing the risks and benefits of allowing nitrates as a food additive in cured meats?
The following information may help to put the issue into a clearer perspective:
- Our major intake of nitrate is from that naturally found in vegetables (86%), with cured meats contributing only 9% and other food commodities the remaining 5% of the nitrate in food.
- Nitrite formed from nitrate in the secretions from the salivary glands (in our saliva) represents the greatest intake of nitrite (77%). Cured meats and other food commodities represent 21% and 2 %, respectively of our nitrite intake.
- Even if nitrite was de-listed as a food additive, we would still be exposed to a substantial intake of nitrite due to its presence in saliva.
- It has also been shown that nitrosamines are formed in the human stomach even when the diet does not contain any nitrite because of the conversion of salivary nitrate to nitrite by bacteria in our mouths.
- The pH and temperature of the human stomach are in the optimum range for nitrosation reactions.
- Consequently, humans have been exposed to nitrosamines for eons of time.
- It is extremely difficult to quantify the risk posed to our health by the use of nitrites as a food additive. The risks appear to be very low.
- On the other hand, the risk of botulism from cured meats if nitrites were banned appear to be high, based on information on the incidence of occurrence of Clostridium botulinum spores in raw meats.
The risk/benefit situation related to the use of nitrites in cured meats as follows:
If nitrite is used:
Risks:
- Potential of increased nitrosamine content in the diet. Although some nitrosamines have shown carcinogenicity at higher doses, this does not appear to be a significant risk with moderate consumption.
Benefits:
- Production of cured meat products at a reasonable cost with adequate control of Clostridium botulinum.
If nitrite is not used:
Risks:
- Increased potential for growth and toxin production by Clostridium botulinum in perishable cured meat products under abusive conditions.
- Shelf-stable canned cured meat products would probably not be available because the increased heat treatment required would produce a product with an undesirable texture.
Benefits:
- Decreased risks due to a decreased load of nitrosamines in the diet. The magnitude of this benefit may not be measurable due to the current load of nitrosamines in the diet from other sources and from nitrosamines formed in vivo.
Outcome
Based on the current evidence the benefits of using Nitrites outweighs the risk. However precautionary measures are in place to ensure the safety of the consumers. These precautionary measures include limiting the usage to specific products in which the risk of Clostridium botulinum is greater. The amount of use is regulated and industries are encouraged to use methods to reduce the risk of nitrosamine formation
04.8 Summary of Lesson 4
- There are various Acts and Regulations that apply to food in Canada, to ensure that Canadian consumers have access to a safe, high quality food supply.
- The Health Products and Food Branch (HPFB) of Health Canada establishes regulations and standards (within the Food and Drugs Act and Regulations-FDR)
- The Canadian Food Inspection Agency (CFIA) enforces these regulations and standards (e.g. Consumer Packaging and Labelling Act & Regulations)
- There are 28 divisions within the FDR. For example, Food additives are regulated (found) in Division 16.
- There are more than 400 approved food additives in Canada. Aspartame and nitrites are two examples of food additives; however, aspartame is classified as a "sweetener", whereas nitrites are classified as "preservatives".
- Generally, risk/benefit issues are not black and white; often they are shrouded in shades of gray. However, with the information you have gained in this course, you should be able to determine objectively the validity of reports that you may encounter in the media about food additives and about food safety issues.
Authorship:
FNH 200 Course content on this wiki page and associated lesson pages was originally authored by Drs. Brent Skura, Andrea Liceaga, and Eunice Li-Chan. Ongoing edits and updates are contributed by past and current instructors including Drs. Andrea Liceaga, Azita Madadi-Noei, Nooshin Alizadeh-Pasdar, and Judy Chan.
|
|