Course:MEDG550/Student Activities/Waardenburg Syndrome Type I
Waardenburg Syndrome Type I (WS1), is a genetically inherited type of syndromic hearing loss. Other features of this condition include pigmentation abnormalities and dystopia canthorum (seemingly widely spaced eyes). WS1 occurs in about 1:20,000 to 1:40,000 live births, comprising 3% of all deaf children [1].
Clinical Features
P. J. Waardenburg first described WS1 in 1951, highlighting key features of the condition that remain part of the clinical diagnostic criteria today [2][3]. In order for an individual to be diagnosed as affected, they must meet two major criteria or one major plus two minor criteria [3][1].
Major Criteria
- Congenital sensorineural hearing loss
- Iris pigmentation abnormalities:
- Two eyes of different color
- An eye with two different colors (iris bicolor/segmental heterochromia)
- Characteristic brilliant blue iris
- Hair hypopigmentation:
- White forelock: can be present at birth and then disappear later in life, with reappearance in teens or adulthood, or may appear for the first time at any age
- Body hair: white hairs within eyebrow, eyelashes, or at other sites on the body
- Eyes that seem widely spaced apart due to the sideways (lateral) displacement of the inner corners (canthi) of the eyes (this is called "dystopia canthorum", or lateral displacement of inner canthi, with a reduction of visible sclera medially)
- First-degree relative previously diagnosed with Waardenburg Syndrome
Minor Criteria
- Several areas of hypopigmented skin (congenital leukoderma)
- Eyebrows that connect in the middle
- Broad, high nasal root (space at the top of the nose and between the eyes is broad)
- Underdeveloped outer cartilage of the nostrils (hypoplasia of alae nasi )
- Premature graying of the hair, occurring before 30 years of age
Inheritance
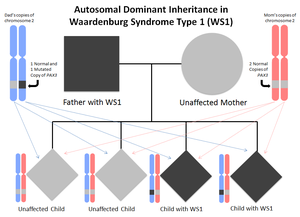
WS1 is inherited in an autosomal dominant manner. This means that each individual only needs to inherit one mutated copy (either from their mother or their father) of the causal gene to be affected by WS1. Accordingly, most affected individuals have an affected parent. In a small subset of affected individuals, there will be no family history of the condition, as the mutation is newly occurring in just that individual (this is called a de novo mutation).
Every individual has 2 copies for every gene that makes up their genome. One copy was passed down from their father in their father’s sperm, and one copy was passed down from their mother via the egg. Accordingly, when we make either sperm or eggs, we pass one of each copy of our genes into the egg or sperm, and which copy the egg or sperm receives is random. Thus, for those affected, because there is a 50% chance that the egg/sperm receives either the normal or the mutated copy of the causal gene, there is a 50% chance of passing the condition on and having an affected offspring.
Genetics
WS1 is caused by mutation in the PAX3 gene (OMIM# 606597), located on the long arm of chromosome 2 at position 36.1 [1]. PAX3 belongs to the PAX family of transcription factors, which are active in neural crest cells during embryonic development, directing differentiation of neural crest cells into other cell types including nerve, craniofacial bones, and pigment-producing melanocytes [4].
The absence of melanocytes in skin, hair and the inner ear during development that occurs with mutations of the PAX3 gene is responsible for the features of WS1 [4]. PAX3 has been shown to have interactions with SOX10, and MITF, explaining the overlap of phenotypes between the different types of Waardenburg Syndrome (see “Differential Diagnoses” below) [1].
Differential Diagnoses
Due to similarities in clinical features, it may be difficult to differentiate WS1 from Waardenburg Syndrome Type II (WS2). WS1 can be distinguished from WS2 by the presence of dystopia canthorum, which occurs only in WS1 [1]. White forelock, leukoderma, high nasal root and medical eyebrow flare are also more prevalent in WS1 than WS2, although sensorineural hearing loss and heterochromic iridum are more prevalent in WS2 [1]. WS2 is caused by mutations of MITF or SOX10 [1].
Waardenburg Syndrome Type III (WS3), in contrast, has dystopia canthorum and many of the other features of WS1, but also features upper limb abnormalities including contractures of the limb muscles or joints, carpal bone fusion, or syndactyly [1][5]. Similar to WS1, WS3 is also caused by mutations in PAX3 [1][6].
Waardenburg Syndrome Type IV (WS4), has the additional feature of Hirschsprung disease on top of pigmentation abnormalities and hearing loss [1][5]. WS4 is caused by mutations in EDNRB, EDN3, or SOX10 [1].
Genotype-Phenotype Correlations
To date, genotype/phenotype correlations in the PAX3 gene are not perfectly characterized. Some observations have nonetheless been made in the scientific literature:
- An Asn→His mutation at position 47 is an established pathogenic variant in WS3 [1][6].
- PAX3 pathogenic variants that delete the homeodomain (as opposed to pathogenic variants that include missense mutations or deletions in the paired domain) are associated with pigmentation disturbance [1].
- presence of eye pigment abnormalities were more strongly associated with individuals carrying deletions of the PAX3 homeodomain and the Pro-Ser-Thr-rich region compared to individuals with an amino acid substitution in the homeodomain [7].
- No genotype-phenotype correlation for the hearing loss in WS1 has been found [1].
- Size of the PAX3 gene deletion does not appear to be predictive of gene severity [1].
Genetic Counselling
Because WS1 is inherited in an autosomal dominant manner (see “Inheritance” above), this means that the majority of individuals affected by WS1 tend to have an affected parent, and that they also have a 50% chance of passing the condition on to their own offspring. Accordingly, siblings of an affected individual with an affected parent have a 50% chance of being affected as well.
In a small proportion of cases, the affected individual will not appear to have an affected parent. This has several implications, both medical and non-medical:
- For the affected individual, the causative PAX3 mutation arose spontaneously in either the sperm or egg that their mother or father provided. In other words, the parents of the affected individual are not likely to be carriers of the PAX3 mutation, and they are unlikely to have more children who are similarly affected.
- There is also a possibility of alternate paternity or maternity (if there was assisted reproduction involving egg donation, as an example).
- That the affected individual has been adopted, and for whatever reason, this has not been explicitly communicated.
If a family affected by WS1 has had genetic testing and the particular PAX3 variant that is being passed down has been identified, then prenatal testing is an option, should the parents which to determine if a fetus is affected by WS1 or not. It’s important to note, however, that knowing the PAX3 mutation status doesn’t necessarily mean the severity of the baby’s symptoms can be predicted. WS1 is extremely variable from one individual to the next, with symptoms ranging from mild to moderate. This includes hearing loss, which may or may be present in different affected individuals belonging to the same family.
Resources
For more information on Waardenburg Syndrome and other forms of hereditary hearing loss, visit:
- NCBI Genes and Disease: Waardenburg Syndrome Type I http://www.ncbi.nlm.nih.gov/books/NBK1531/
- NCBI Genes and Disease: Deafness and Hereditary Hearing Loss Overview http://www.ncbi.nlm.nih.gov/books/NBK1434/
To connect with others affected by Waardenburg Syndrome, and access other resources, visit:
- Waardenburg Syndrome Support Group: http://www.dailystrength.org/c/Waardenburg-Syndrome-WS/support-group
- Canadian Association for the Deaf: http://www.cad.ca/
- National Organization of Albinism and Hypopigmentation (NOAH): http://www.albinism.org
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 GeneReviews®, Milunsky JM. Waardenburg Syndrome Type I. 2001 Jul 30 [Updated 2014 Aug 7]. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2015.
- ↑ Waardenburg PJ. A New Syndrome Combining Developmental Anomalies of the Eyelids, Eyebrows and Nose Root with Pigmentary Defects of the Iris and Head Hair and with Congenital Deafness - Dystopia-Canthi Medialis Et Punctorum Lacrimalium Lateroversa, Hyperplasia Supercilii Medialis Et Radicis Nasi, Heterochromia Iridum Totalis Sive Partialis, Albinismus Circumscriptus (Leucismus, Poliosis), Et Surditas Congenita (Surdimutitas). Am J Hum Genet 1951;3(3):195-253.
- ↑ 3.0 3.1 Farrer LA, Grundfast KM, Amos J, Arnos KS, Asher JH, Beighton P, et al. Waardenburg Syndrome (Ws) Type-i is Caused by Defects at Multiple Loci, One of which is Near Alpp on Chromosome-2 - 1st Report of the Ws Consortium. Am J Hum Genet 1992 MAY;50(5):902-913.
- ↑ 4.0 4.1 Genetics Home Reference, National Library of Medicine (US). Genetics Home Reference [Internet]. Bethesda (MD): The Library; 2015 Mar 10. PAX3; [reviewed 2012 Aug; cited 2015 Mar 12]. Available from: http://ghr.nlm.nih.gov/gene/PAX3
- ↑ 5.0 5.1 Online Mendelian Inheritance in Man, OMIM®, Johns Hopkins University, Baltimore, MD. MIM Number: {193500}: {04/03/2012}: . World Wide Web URL: http://www.omim.org/entry/193500
- ↑ 6.0 6.1 HOTH C, MILUNSKY A, LIPSKY N, SHEFFER R, CLARREN S, BALDWIN C. Mutations in the Paired Domain of the Human Pax3 Gene Cause Klein-Waardenburg Syndrome (Ws-Iii) as Well as Waardenburg Syndrome Type-i (Ws-I). Am J Hum Genet 1993 MAR;52(3):455-462.
- ↑ DeStefano A, Cupples L, Arnos K, Asher J, Baldwin C, Blanton S, et al. Correlation between Waardenburg syndrome phenotype and genotype in a population of individuals with identified PAX3 mutations. Hum Genet 1998 MAY;102(5):499-506.