Course:MEDG550/Student Activities/Familial Mediterranean Fever
Familial Mediterranean Fever (FMF), also known as recurrent polyserositis or benign paroxysmal peritonitis, is a genetic condition characterized by recurrent episodes of fever and inflammation.[1] FMF is classified as an autoinflammatory disorder, a group of conditions, also known as periodic fevers that are characterized by recurrent periods of inflammation and fever. [2] FMF is caused by genetic changes in a single gene called MEFV.
Clinical Features
There are two clinical presentations of FMF, Type 1 and Type 2.
Familial Mediterranean Fever Type 1
Individuals with FMF Type 1 have recurrent episodes of inflammation and fever, which develop spontaneously [3] and can last between 1-4 days.[4] The inflammation affects the linings of the lungs, abdomen, joints and, rarely, the heart and brain.[3][5] Fevers are unaffected by antibiotics and can be as high as 38-40°C.[4] More than 90% of individuals with FMF will experience abdominal pain during an episode of inflammation, and patients can also experience joint pain, such as arthritis, and chest pain from inflammation of the lungs or heart.[3][4] The episodes of fever and inflammation can occur as frequently as once a week or only once every few years[4] and the type and severity of symptoms varies between individuals.[5]
If FMF is left untreated, amyloidosis can develop.[5] Amyloidosis is the build up of the protein amyloid in the kidneys and is the most serious complication of FMF as it can lead to renal failure. [6]
Familial Mediterranean Fever Type 2
Individuals with FMF Type 2 present with amyloidosis as their first clinical feature and otherwise do not show any symptoms.[5]
Prevalence
As the name suggests, FMF is found predominantly in people of Mediterranean descent, specifically Armenian, Turkish, Arabic, or Jewish (including North African, Iraqi, and Ashkenazi Jewish).[7] In these populations, the prevalence of individuals with FMF ranges from 1/200 to 1/1000.[4] [7] FMF has also been reported in individuals of Greek, Spanish, Italian and Japanese descent.[7]
Diagnosis
The vast majority of patients (up to 90%) are diagnosed before the age of 20, with more than half showing symptoms before the age of 10.[3] Diagnosis is clinical, based on the Tel Hashomer clinical criteria.
Tel Hashomer Clinical Criteria[8]
- Fever and
- 1 additional major feature and one minor feature, OR
- 2 minor features
Major Features
- fever
- abdominal pain
- chest pain
- joint pain
- skin eruption, for example a rash
Minor Features
- increased erythrocyte sedimentation rate (ESR) (a measure of how quickly red blood cells stick together)
- leukocytosis (high white blood cell count)
- elevated serum fibrinogen concentration (the protein fibrinogen causes the red blood cells to stick together)
Patients can also be diagnosed through molecular diagnosis. This can be done by looking at the sequence of the MEFV gene to identify any abnormal changes, or to look for specific gene changes known to be associated with specific ethnicities or families.[8]
If there is no conclusive diagnosis, an individual with suspected FMF can be treated with colchicine, an oral medication, for a trial period of 6 months to see if their symptoms improve with the medication.[8]
Treatment and Management
Treating FMF is aimed at reducing the frequency of the fever and pain, and controlling chronic inflammation between episodes to reduce the accumulation of amyloid in the kidneys.[9] The most effective medication for FMF is a drug called colchicine. Colchicine prevents inflammatory attacks and amyloid deposition.[9] Patients with severe FMF symptoms and the most pathogenic mutations may take high doses of colchicine daily, while some patients with less severe symptoms and mutations that are less associated with complications such as amyloidosis may only take colchicine during an inflammatory attack.[8]
In patients with amyloidosis, they can require dialysis or even a kidney transplant, as the accumulation of amyloid can reduce kidney function and lead to renal failure.[9]
Genetics and Inheritance
FMF is a genetic condition caused by mutations in the MEFV gene. Normally, the MEFV gene has instructions for creating a protein called pyrin, which is responsible for regulating the inflammatory response.[8] In individuals with FMF, pyrin becomes less functional, leading to the increased inflammatory responses.[8]
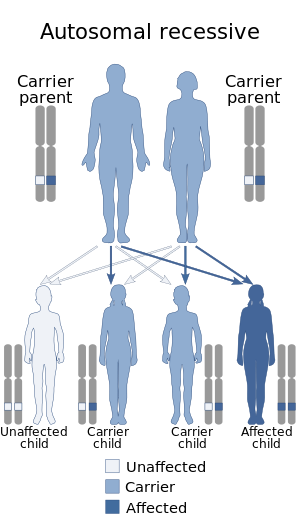
FMF is inherited in an autosomal recessive manner, meaning that an individual with FMF has inherited two copies of the altered MEFV gene, one from each parent. A person who has only one altered copy of the MEFV gene is said to be a "carrier" of FMF and generally shows no symptoms. There have been rare cases where a person can have only altered copy of the MEFV gene and show symptoms of FMF [10]. These individuals often have gene changes in their altered copy that are more severe, and will have later onset and milder symptoms than someone with both copies altered.[10] In these cases, FMF can be inherited in an autosomal dominant pattern, where only one mutated copy of MEFV inherited from an individual's parents is enough to show symptoms.[10]
Other cases of FMF may appear to be autosomal dominant, but are actually “pseudodominant.” This can occur when a child is born to one parent with FMF and another parent who has FMF but does not show symptoms, creating the appearance of an autosomal dominant inheritance pattern.[11] This “pseudodominance” can be clarified by molecular testing of both parents of an affected individual.
Genetic Counselling
Individuals who have FMF, a family history of FMF, or are a member of one of the at-risk ethnicities can attend a genetic counselling session to learn more about the condition, as well as to discuss carrier testing and prenatal diagnosis options if applicable.
Parents of a child with FMF can meet with a genetic counsellor to discuss the chance of having another child with the condition. As FMF is generally inherited in an autosomal recessive manner, the chance of another child with FMF is 25%. As some cases of FMF are inherited in an autosomal dominant pattern, the genetic status of the parents can be important to know to clarify cases of pseudodominance and discuss the exact risk for each family.[12]
If the exact variants of the MEFV gene are known in a particular family, there is the option of carrier testing for at-risk relatives and also prenatal diagnosis such as amniocentesis or chorionic villus sampling in subsequent pregnancies.[8] Chorionic villus sampling (CVS) involves getting a sample of the placenta through the cervix or through the abdomen around 11-13 weeks in pregnancy, while amniocentesis involves inserting a needle through the abdomen to get amniotic fluid samples after 15 weeks in pregnancy. In both methods, the samples can be sent to a laboratory, where genetic testing can be performed to see if the baby carries any abnormal MEFV gene changes. However, both methods come with a small risk (0.5-1%) of miscarriage or premature labour.
Genetic testing can also be done before a pregnancy even begins. Couples who are identified as carriers and wish to have children without the abnormal MEFV gene variation can choose to conceive through in vitro fertilization (IVF), where sperm and eggs are retrieved from the parents and are fertilized in the lab to create embryos. Some cells can be taken from each embryo, and a process called pre-implantation genetic testing for monogenic disorders (PGT-M) can be used to determine whether the embryo carries the mutations. The couple can preferentially select for embryos that do not have the mutations.
It can be important to test siblings, parents, and children of an affected individual, even if they appear asymptomatic. Cases of FMF Type 2 show amyloidosis as the first symptom, and so individuals who have a molecular diagnosis of FMF can begin preventative treatment with colchicine.[12]
Genotype-Phenotype Correlations
There have been over 250 mutations identified in the MEFV gene[13] and specific changes in MEFV are associated with differences in symptoms and severity. This is termed as genotype-phenotype relationship, where different genetic changes show a different clinical presentation.
For example, the M694V mutation is associated with the development of amyloidosis[12] and patients with this mutation in both copies of the MEFV gene can be less responsive to colchicine treatment.[14] The notation 'M694V' represents when one amino acid in the protein has been changed to another at a particular location. Individuals who have both copies of the MEFV gene with the M694V mutation tend to have an earlier age of onset and have more symptoms of arthritis and joint pain.[15] Other examples include the mutation T577N, which has been associated with autosomal dominant FMF[16] and E148Q, which has been argued to cause a mild form of FMF in some individuals.[5]
Living with Familial Mediterranean Fever
Chronic conditions such as FMF can pose a serious burden and challenge on affected individuals and their family members. Chronic conditions were shown to result in increased psychological and emotional distress, family issues, financial issues, and social isolation; also decrease in physical and social activities in families [17].
Considerations for individuals affected with FMF:
Patients diagnosed with FMF can experience frequent episodes of fever and fatigue. Patients frequently reported not being able to keep up with their roles such as being a spouse, parents, student, or worker during these FMF episodes[18]. Below are experiences of patients with FMF:
- Affected males often reported difficulties in keeping up with finances, whereas females reported difficulties in taking care of their children and husband[18].
- As episodes can occur anywhere and anytime, many patients reported feeling the most anxious about the uncertainty of the timing of their next episode[18].
- As affected individuals are healthy and well between episodes of FMF patients reported not being understood by their friends, family, and coworkers about their condition and were accused of role-playing their symptoms[18].
- Patients were found to have higher anxiety and depression levels than the general population[19]. Anxiety and depression should be evaluated and treated in these patients to improve their overall health.
- Adult patients also reported having more dissatisfaction with various aspects of their life in terms of their health, job, being independent, and relationships with friends and family[20].
- Kids with FMF missed more school days than the general population[20]. Although kids might miss school during episodes, once their symptoms were controlled with the treatment they were able to continue their education without any interference[20].
- There was no difference in terms of the number of times kids with FMF visited the emergency room or family doctor[20].
- Patients with FMF reported having low quality of life due to physical, social, and emotional implications of the condition[21]. Several health factors including disease symptom severity, pain, depression, sleep patterns, and treatment can play a role in the assessment quality of life[21].
Uncertainty and challenges of FMF can have serious implications for individuals. Affected patients can benefit from additional support and effective coping strategies for a better quality of life.
Considerations for parents and caregivers:
Parents and caregivers of FMF might experience high stress, fear, anxiety, and burden[22]. They might have to quit working or reduce their work hours to take care of their child[22]. They might feel lonely and isolated, guilty for reduced income which may result in self and family conflicts[22].
- Mothers of FMF patients reported being less satisfied with their lives compared to mothers of healthy children[20]. No reported difference was found in fathers[20].
- Mothers of FMF children were found to have higher anxiety and depression levels compared to their husbands[20]. Many mothers were worried about the uncertainty of the disease prognosis and outcome[20].
- Caregivers and parents might feel overwhelmed and seek out coping strategies. Caregivers were usually found to rely on unhealthy coping strategies such as substance use, denial, self-blame, and self-distraction, especially when FMF symptoms weren't managed well[22].
FMF is a chronic condition and patients and their caregivers should be provided with appropriate social, economic, physical, and psychological support. A multidisciplinary team of psychologists, social workers, and other health care professionals are an important part of managing FMF symptoms and supporting caregivers in coping with their burden[22].
Patient Resources
The Canadian Amyloidosis Support Network
Systemic Autoinflammatory Disease (SAID) Support
References
- ↑ Ben-Chetrit E, Touitou I. (2009). Familial Mediterranean Fever in the World. Arthrit Care Res 61(10):1447-1453.
- ↑ Ciccarelli F, De Martinis M, Ginaldi L. (2013). An Update on Autoinflammatory Diseases. Curr Med Chem 21(3):261-269.
- ↑ 3.0 3.1 3.2 3.3 Sari I, Birlik M, Kasifoglu T. (2014). Familial Mediterranean fever: an updated review. Eur J Rheumatol" "'1'(1):21-33.
- ↑ 4.0 4.1 4.2 4.3 4.4 Manna PR. Familial Mediterranean Fever. Orphanet encyclopedia, January, 2012, http://www.orpha.net/consor/www/cgi-bin/OC_Exp.php?lng=EN&Expert=342
- ↑ 5.0 5.1 5.2 5.3 5.4 Shohat M, Halpern GJ. (2011). Familial Mediterranean fever – a review. Genet Med 13:487-498.
- ↑ Familial Mediterranean Fever. Genetic and Rare Diseases Information Center, January, 2012, https://rarediseases.info.nih.gov/diseases/6421/familial-mediterranean-fever/cases/33603
- ↑ 7.0 7.1 7.2 Gattorno M. Familial Mediterranean Fever. Springer International Publishing Switzerland; 2015.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 Shohat, M. 2000 Aug 8 [Updated 2016 Dec 15]. Familial Mediterranean Fever. Gene Reviews. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1227/
- ↑ 9.0 9.1 9.2 Ozen S, Demikraya E, Erer B et al. (2016). EULAR recommendations for the management of familial Mediterranean fever. Ann Rheum Dis 75:644-651.
- ↑ 10.0 10.1 10.2 Shohat, M. 2000 Aug 8 [Updated 2016 Dec 15]. Familial Mediterranean Fever. Gene Reviews. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1227/
- ↑ Shohat, M. 2000 Aug 8 [Updated 2016 Dec 15]. Familial Mediterranean Fever. Gene Reviews. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1227/
- ↑ 12.0 12.1 12.2 Ben-Chetri E, Sagi M. (2001). Genetic counselling in familial Mediterranean fever: has the time come? Rheumatology 40(6):606-609.
- ↑ Sari I, Birlik M, Kasifoglu T. (2014). Familial Mediterranean fever: an updated review. Eur J Rheumatol" "'1'(1):21-33.
- ↑ Soylemezoglu O, Arga M, Fidan K, et al. (2010). Unresponsiveness to colchicine therapy in patients with familial Mediterranean fever homozygous for the M694V mutation. J Rheumatol 37:182–189.
- ↑ Tunca M, Akar S, Onen F, et al. (2005). Familial Mediterranean fever (FMF) in Turkey: results of a nationwide multicenter study. Medicine (Baltimore) 84:1–11.
- ↑ Stoffels M, Szperl A, Simon A, et al. (2014). MEFV mutations affecting pyrin amino acid 577 cause autosomal dominant autoinflammatory disease. Ann Rheum Dis 73:455–61.
- ↑ Kosan, Zahide (2019). "Evaluation of the Burden of Care and the Quality of Life in the Parents of Turkish Children with Familial Mediterranean Fever". Pediatr Nurs.
- ↑ 18.0 18.1 18.2 18.3 Yuksel, Cigdem (2019). ""Reality of living with familial mediterranean fever identity": A phenomenological study". Gulhane Medical Journal. 61.
- ↑ Sag, Sinem (2018). "Frequency of depression, anxiety, and fatıgue in fmf patients and their association with
disease parameters". Medicine Science. line feed character in
|title=at position 89 (help) - ↑ 20.0 20.1 20.2 20.3 20.4 20.5 20.6 20.7 Press, J. (2000). "Living with a child with familial Mediterranean fever: Does it affect the quality of life of the parents ?". PEDIATRIC RHEUMATOLOGY.
- ↑ 21.0 21.1 Guler, Tuba (2018). "Quality of life in Turkish patients with Familial Mediterranean Fever: Association with fatigue, psychological status, disease severity and other clinical parameters". The Egyptian Rheumatologist. 40.
- ↑ 22.0 22.1 22.2 22.3 22.4 Yildirim, Deniz Gezgin (2021). "Evaluation of caregiver burden and coping strategies in parents of paediatric familial Mediterranean fever patients in relation to illness severity, therapy and health-related quality of life". Quality of Life Research. 30.