Course:MATH110/Archive/2010-2011/003/Teams/Solothurn/Homework 13
Homework 13: Team Problem
pH: The Power of Understanding
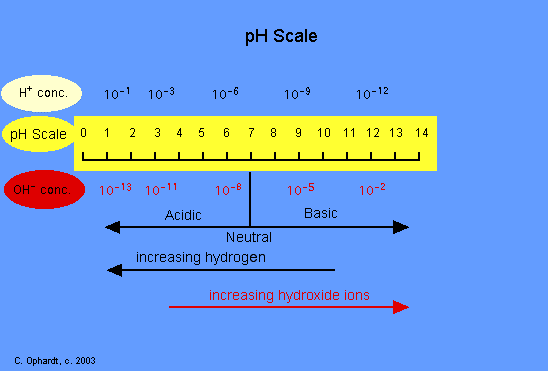
The pH scale measures acidic and basic chemical properties. The scale ranges from 0 to 14, with a pH of 7 being neutral. Any pH less than 7 is acidic, whereas a pH of greater than 7 is basic. The pH scale is a logarithmic scale, which means that each lower numerical value is ten times more acidic than the next higher numerical value (eg. pH of 3 is ten times more acidic than a pH of 4, and 100 times (10 times 10) more acidic than a pH of 5). This property works for basic values as well, with each higher value being ten times more alkaline, or basic, than the value below it (eg. pH of 9 is ten times more alkaline than pH of 8).
pH is a measure of the concentration of hydrogen ions. The definition of pH is the negative logarithm of a hydrogen ion concentration. The less ions that are present, the more alkaline the solution. If more hydrogen ions are found the more acidic. To find the pH, we need to find the logarithm of the H+ (hydrogen) concentration, and then reverse the sign - the only case in which the sign is not reversed is if the H+ concentration is greater than 1 M (which is highly unusual).
The fact that the pH scale is a logarithmic scale turns out to be very useful when scientists need to calculate very high values of hydrogen ions in solutions. In fact, strongly basic solutions might have, let’s say, 100.000.000.000.000 times less hydrogen ions than strongly basic solutions. It is then obvious that dealing with such large numbers is a source of complications for the scientists that need to analyze this data. A logarithmic scale helps scientists deal with extremely large numbers for pH in a more efficient way, with applications in various fields such as marine biology and oceanography (sea water), medicine (body fluids) and certainly chemistry.
For comparison purposes, the pH scale uses logs because logs can expand the values between 0 and 1 on a compact scale to make it into a large scale, this also means that the log scale changes. pH scale uses logs when the answer or the change in the values depends on the size of the the change in proportion to the value and not on the absolute size of change. For example, when you graph two acids, one with a concentration of 0.001 and one with a weaker acid say with pH4 as opposed to the first, pH3, it would be 0.0001. When you graph this, the change barely shows and it comes up as the same value, implying that all neutral and basic values are indistinguishable. Since it is important to show the difference between the two, a weak and a weaker acid, then these values are needed, and therefore we use logs to find these values to plot on our graph.
The equation of pH is:
pH = -log [H+]
eg. what is the pH of a substance with an H+ concentration of 0.001?
to find the pH, we first should convert the concentration value into exponential notation:
0.001M = 10^-3
we than plug this number into our formula of pH = -log [H+]
pH = -log [10^-3]
pH = 3
This means that since pure water is pH 7
The concentration of hydrogen ions would be 0.0000001, plugged into the pH formula, it would look like pH = -log [10^-7]
FEEL FREE TO CHECK OUT THE YOUTUBE VIDEO FOR A VISUAL EXPLANATION!
sources: http://www.elmhurst.edu/~chm/vchembook/184ph.html http://www.science.uwaterloo.ca/~cchieh/cact/c123/ph.html http://mathforum.org/library/drmath/view/55574.html