Bacterial mortality in aquatic environments
Written by: Yvonne Yiu and Angel (Huan Wen) Xu
The microbial community is now recognized as a significant contributor towards the cycling of energy and nutrient within aquatic environments [1]. In the field of marine microbiology, many studies have focused on the various factors affecting the rate of bacterial production and the nutrient fluxes within the ‘microbial loop’ [2]. As a substantial amount of carbon biomass is assimilated from bacteria and transferred to higher trophic levels [2], such transfers have been characterized in different environments. To measure bacterial standing stocks and production, direct and indirect methods have been developed, such as quantifying the frequency of dividing cells and the incorporation of nucleic acid or amino acid precursors by bacterial cells [3][4]. However, while bacterial production is important in understanding the structure and functioning of the marine food web, the causes and consequences of bacterial mortality also has significant impact on energy flow and nutrient cycles.
The majority of bacterial biomass was once thought to be consumed by protist grazing [5] [6]. Even so, grazing estimates could not entirely account for bacterial mortality rates observed, and hence, residual losses were attributed to unknown mechanisms [7]. More recently, however, viruses have not only been found in high abundances in the marine ecosystem, but they have also been demonstrated to act as a dynamic population that interacts with the bacterial community [8][9]. For instance, 23% of total viruses were found to be attached to dead or senescent diatoms during the collapse of a spring bloom in western Norway [10].
Hence, studying the relative importance of both protist grazing and viral lysis in bacterial mortality may provide insight underlying the forces that drive the marine community [11] .
Background
Protist Grazing
In most communities, bacterial mortality is partially attributed to protistan grazers, including flagellates and ciliates [12]. Compared to the sloppy feeding habits of copepods, grazing by protists is highly efficient, with little organic matter released into the water column [12]. However, the specific mechanisms in particle uptake prevent protists from being indiscriminate feeders on all bacterial shapes or cell size classes [12]. Small heterotrophic flagellates, which are thought to be responsible for over 80% of marine bacterivory, prefer to ingest bacterial cells ranging from 1 to 3um [13]. The diversity of the protistan community varies depending on the ecosystem’s productivity; heterotrophic flagellates tend to thrive in eutrophic and oligotrophic waters, whereas higher numbers of ciliates are found in nearshore and estuarine environments [14]. Bacterial densities within a community can also affect protist grazing, as concentrations below the threshold will limit the rate of predation, and ciliates and flagellates cease growth [15]. Grazing effects on bacterial mortality can be measured through selective filtration to eliminate predators [5], the dilution method [16], antibiotic use to inhibit grazing effects [17], as well as rate of loss of DNA from cells pre-labelled with tritiated thymidine [18]. Fluorescently labeled bacteria (FLB) can also be used as tracers to obtain the clearance rates, as well as specific clearance estimates to measure protist counts or biovolume [19]
Viral lysis
Viruses typically proliferate and induce death of the host cell through lytic or lysogenic infections. In lytic replication, viruses take advantage of host mechanisms and redirect them to produce and release viral progeny after cell lysis, while lysogenic infections occur when a virus integrate their genome into the host chromosome, and induce cell lysis upon a cellular or environmental signal [20]. However, host specificity and contact rate limit the ability of viruses to infect marine bacteria, as viruses can only infect a small taxonomic group of hosts, and contact is affected by both viral and bacterial concentrations in the environment [12]. The presence of viruses can be detected through transmission electron microscopy (TEM), but the fraction and time of the infection cycle, as well as cell generation time must be considered [21]. In other methods, an estimate of burst size is required to calculate the proportion of bacterial mortality attributed to viruses. These methods include viral decay rate by cyanide, virus production estimated from radioisotope incorporation, and fluorescently labeled viruses, in which loss and production rates can be measured by the total counts and proportion of labeled viruses in the sample [19]. Though an indirect method, the disappearance of bacterial DNA can also measure mortality rate in samples subjected to size fractionation to remove protists [18].
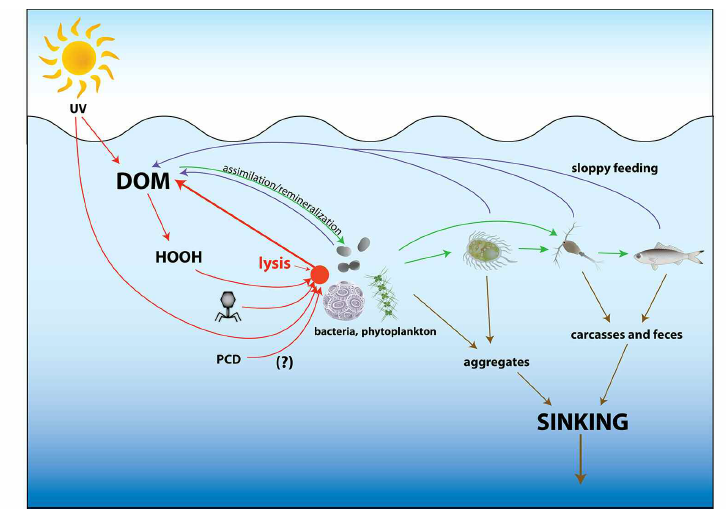
Viruses and protists cause similar bacterial mortality in coastal seawater
Fuhrman, JA, Noble, RT. 1995. Viruses and Protists Cause Similar Bacterial Mortality in Coastal Seawater. Limnol. Oceanogr. 40:1236-1242.
Aim
Fuhrman and Noble (1995) evaluated bacterial production and loss in the coastal seawater obtained from Santa Monica, California. They used three independent methods, size fractionation of labeled DNA disappearance, disappearance of fluorescently labeled bacteria (FLB), and virus production, to measure the contribution of bacterial loss by protist grazing and viral lysis. The results from all three independent methods are relatively consistent and all suggest that protist grazing and viral lysis caused similar bacterial mortality rates in this system. This study suggests that virus is an important player in marine food web and that viral activity should be considered in marine bacterial process studies.
Results
Sample were collected from Santa Monica Pier and then transported to the lab. Measurements on mesocosm 1 started on June 15, 1993 and mesocosm 2 was started on November 17, 1993.
Total loss rate
At each time point, a portion of the sample was aliquoted into polypropylene tubes, which are then inoculated with [3H]thymidine or [3H]leucine. Thymidine or leucine incorporation were converted to bacterial production using the conversion factor of 2 x 1018 cells produced per mole of thymidine incorporated[22] and 1.5 x 1017 cells produced per mole of leucine incorporated[23][24]. Bacterial loss is then calculated using the equation Loss = production – (Nt – No), where Nt is the final bacterial abundance and No is the initial bacterial abundance over the time period when bacterial production and loss are measured. Bacterial production measured by thymidine and leucine was similar, and is assumed to be accurate. Bacterial production was measured to be 4.7% h-1 in mesocosm 1 and 7.9% h-1 in mesocosm 2, whereas bacterial loss was calculated to be 4.4±0.3% h-1 in mesocosm 1 and 8.3±0.8% h-1 in mesocosm 2.
Size fractionation of labeled DNA disappearance
Bacterial DNA was first “pulse-labeled” by adding low levels of [3H]thymidine to the sample, and the incorporation peak was determined using radioactivity. Next, half of the sample was filtered using 1.0 μm pore size filter for mesocosm 1 and 0.6 μm pore size filter for mesocosm 2 to remove protists. The filtered and unfiltered sample was incubated side by side and bacterial mortality rate was determined by decline of radioactivity. In mesocosm 1, bacterial loss was determined to be 2.4±0.2% in the unfiltered sample where as in the filtered sample with protists removed, the loss was 1.2±0.2%, which is a two-fold decrease compared to the unfiltered sample. In mesocosm 2, 75% of the labels were removed during filtration, and estimates were only based on two data points, so the numbers are questionable. However, this method of estimating bacterial mortality is expected to produce an underestimate of the true bacterial mortality rate as bacterial death does not necessarily mean degradation of its DNA, and indeed, the bacterial loss determined using is method is lower than the number estimated using [3H]thymidine and [3H]leucine.
Disappearance of Fluorescently Labeled Bacteria (FLB)
Bacteria from Santa Monica Bay seawater were heat killed and stained with fluorescein to produce FLB. FLB were then added to samples taken from mesocosm and FLB concentrations were measured every 6-8 hours. Since FLB were heat killed, their removal rate by protists is underestimated, so a correction factor of 2 has been applied in the calculation. The bacterial loss estimated from the corrected FLB was ~2% h-1 in mesocosm 1 and ~3.1% h-1 in mesocosm 2.
Virus production
Virus production was estimated using [3H]thymidine incorporation and the conversion factor of 6.17 x 1020 viruses produced for every mole of thymidine incorporated is used to determine the viral production rate. Viral production in mesocosm 1 was found to be 1.78±0.12 x 109 and 1.61±0.11 x 109 viruses L-1 h-1, and 1.05±0.07 x 109 and 1.03±0.05 x 109 in mesocosm 2. Burst size was estimated from TEM observations to convert viral production to bacterial loss, and the burst size in these samples was found to be ~20 viruses. From this, bacterial loss from viral lysis was estimated to be 1.3% h-1 in mesocosm 1 and 2.8% h-1 in mesocosm 2.
Conclusion
Summing up the bacterial loss estimated from FLB disappearance and viral lysis, the two account for 75% of the total bacterial loss as estimated from [3H]thymidine and [3H]leucine incorporation in mesocosm 1 and 71% in mesocosm 2. Of the 75% in mesocosm 1, protist grazing estimated from FLB accounts for 61% of the bacterial loss and viral lysis accounts for 39%, and of the 71% in mesocosm 2, protists account for 53% of the loss and viral lysis accounts for 47% of the loss. From this, the authors have concluded that grazing and viral lysis accounts for 70-75% of the bacterial mortalities and these two processes cause similar bacterial mortality rates.
Critical Analysis
Weakness
As the results of this study were heavily relied on calculations, uncertainties arise when assumptions and estimates were used in these calculations. For example, since protists preferentially graze on fast-moving bacteria [25] [26] compared to non-motile preys, a correction factor must be applied to the calculation of grazing rate as FLBs are heat killed and therefore non-motile. However, as the relative abundance of non-motile, slow-moving, and fast-moving bacteria in the coastal waters of Santa Monica was unknown, the authors had to assume that their abundances were equal and therefore assumed that the correction factor of 2 is reasonable. A change in the correction factor used in the calculation could shift the results of the disappearance of FLB experiment. Also, in the Size fractionation of labeled DNA disappearance experiment, results for mesocosm 2 could not be gathered as the majority of the labels were removed during the filtration process, and therefore cannot support the results from mesocosm 1.
Strength
Previous studies on viral related bacterial loss were mostly based on only one method in measuring viral related bacterial loss [27][21]. However, in this study, Fuhrman et al. used three independent methods to measure the relative bacterial mortality rates caused by protists or viruses, and all three methods gave relatively consistent results.
Personal Opinion
Although there were some weaknesses in the methods used and there is still a lot of disagreements as to the relative bacterial mortality caused by the two groups, protists and viruses, the results of this study still gave strong evidence supporting the significance of viruses in the marine system.
Impact On The Scientific Community
Significance
Following Fuhrman’s & Noble’s (1995) findings, studies delved further into the ecological consequences of both viral lysis in addition to protist grazing on bacterial mortality [11]. Depending on the method of cell death, cell contents can either be transferred to higher trophic levels through protist grazing [20], or be recycled back into the bacterial biomass through viral lysis, which creates a “short-circuit” in energy flow and deprives higher trophic levels of nutrients [10].
Grazers usually exert top-down grazing pressure on bacterial production, as observed in the open-ocean, where the control of picophytoplankton growth lead to the development of high-nutrient, low-chlorophyll regions [28]. From ingestion of bacterial prey, grazers excrete byproducts of metabolism, including ammonium, urea, soluble reactive phosphorus, and dissolved organic matter (DOM) [12]. As these byproducts tend to be in highly labile forms, they are rapidly taken up by phytoplankton or by organisms in higher trophic levels [12]. However, the relative abundance and stoichiometry of excretory products depends upon their chemical composition, refractory material, as well as the taxon or species of both the grazer and their prey[12].
Contrasting from protist grazing, which is only limited by class sizes and surface properties, viruses are host-specific [12]. Moreover, viral lysis is believed to occur at the highest rates in the most abundant hosts [29]. Hence, this decreases host abundance, preventing a specific taxon from dominating the microbial community.
Through viral lysis, bacterial biomass is diverted away from the classical grazing pathway leading up to higher trophic levels; instead, it is released back into the particulate and dissolved pools of organic matter [30]. A large part (6 to 26%) of carbon derived from the bacterial cell remains fixed in surface waters, where it is available to heterotrophic bacteria again, forming a closed loop with respect to organic matter cycling [12]. Nutrients can also be remineralized or broken down photochemically, thus restoring nitrogen and phosphorus concentrations within the local marine environment, and providing labile forms of iron for phytoplankton uptake [31][32]. As viral lysis recycles organic matter from its hosts and make it available for uptake by uninfected bacteria, this promotes the production of surviving bacteria and phytoplankton, thus increasing bacterial biomass and productivity overall.
Another significant effect of viral lysis on bacterial mortality and the marine environment is that before lysis occurs, the virus may have integrated its genome into the host cell’s own genome, thereby being expressed and in some instances, changing the cellular machinery involved in nutrient cycling, carbon metabolism and even photosynthesis [12].
Contradictory Findings
Although both protist grazing and viral lysis have been widely accepted to be major contributors to bacterial mortality observed in the marine environment, there is still much controversy about the relative importance of either factor. Using counts of FIB to measure viral lysis, and protist abundances and clearance rates, findings from Steward et al also showed comparable contributions of both factors on bacterial losses in the Bering and Chukchi Seas, which has similar productivity levels as the Californian coastal waters studied by Fuhrman and Noble (1995) [33][11]. On the other hand, Boras et al studied bacterial mortality in the same marine environment as Fuhrman and Noble (1995), but found that viruses contributed 37% more in bacterial losses than protist grazing over a two-year period [34][11].
While Almeida et al also agree that both factors were able to account for most of the bacterial mortality observed in the brackish and marine waters of Ria de Aveiro, predation was more prominent in both systems [35]. Even more, using similar approaches as Fuhrman and Noble (1995) in estimating bacterial mortality, Guixa-Boixeru et al found that only 11.1 - 21.5% of bacterial mortality was attributable to the viruses in microcosms from the oligotrophic NW Mediterranean [36]. However, the significance of flagellate grazing was measured by the disappearance of fluorescently labelled bacteria from an isolate rather than a natural sample, and viral infection was measured with both percentage of visibly infected bacteria (%VIB) and viral decay rates (VDR).
As these studies are done in different environments using different methods of measurement, difficulties arise when comparing conclusions between the relative importance of protists and viruses in the control of bacterial populations.
Future Directions
Environmental factors
Changes in environmental factors may lead to the varying contributions of protist grazing and viral lysis in bacterial mortality observed in different studies. This was recognized by Bettarel et al, who studied viral activity in both eutrophic and oligotrophic lakes, and found that mortality was more attributed to viruses in less productive systems such in the anoxic hypolimnion [37]. Weinbauer and Holfle also found that within a single eutrophic lake, viruses exerted a greater effect on bacterial mortality in the anoxic hypolimnion, while grazing was the dominant cause of bacterial mortality in the oxic epilimnion [38]. Alternatively, in the bottom of the winter Arctic waters, which is implicated to have low productivity, Wells and Deming observed mortality mortality as primarily attributed to viruses [39]. The dissolved organic matter from viral lysis was recycled back to bacteria, and supported their growth through the winter, though this prevents transfer to higher trophic levels. From these implications, there may be correlations between the type of environment and nutrient conditions with the factors involved in bacterial mortality, which can be analyzed more in depth in future studies.
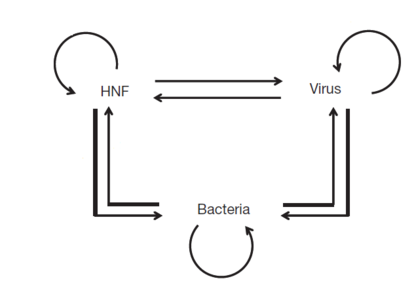
Complexity of interactions
As opposed to an entire bacterial community, Middelboe studied a single virus-host system and found a positive association between bacterial production and the rate of cell lysis and viral production [41]. Moreover, resistant bacteria had an increased growth rate when the viral lysis of sensitive bacteria increased, suggesting the production of dissolved organic matter from viral lysis as promoting the growth of surviving bacterial populations.
In an unrelated study, Simek et al found that flagellate grazing may stimulate viral activity while studying changes in the bacterial community, under enhanced flagellate grazing, as there was an association between viral concentrations and frequencies of infected cells with grazing rates [42]. Moreover, Weinbauer and Holfle also observed a decrease in grazing rates with depth and oxygen concentration, while viral lysis became prominent [38].
These studies suggest among organic and inorganic nutrients, bacteria, viruses and grazing protists, direct and indirect interactions are likely to occur, and have a major impact on the community structure as well as the biogeochemical functions of the other groups [40]. As each of these fates significantly affects the flow of organic matter and individual nutrients into the open ocean, a better understanding on the mode of bacterial mortality will be significant in controlling and characterizing the complexity of the marine food web.
References
- ↑ Azam, F, Fenchel, T, Field, J, Gray, J, Meyer-Reil, L, Thingstad, F. 1983. The ecological role of water-column microbes in the sea. Marine Ecology Progress Series.Oldendorf. 10:257-263.
- ↑ 2.0 2.1 Hagström A, Azam, F, Andersson, A, Wikner, J, Rassoulzadegan, F. 1988. Microbial loop in an oligotrophic pelagic marine ecosystem: possible roles of cyanobacteria and nanoflagellates in the organic fluxes. Mar. Ecol. Prog. Ser. 49:171-178.
- ↑ Hagstrom AU, Horstedt P, Normark S. 1979. Frequency of dividing cells, a new approach to the determination of bacterial growth rates in aquatic environments. Appl. environ. Microbiol. 37: 805-812
- ↑ Fuhrman JA & Azam F. 1980. Bacterioplankton secondary production estimates for coastal waters of British Columbia. Antarctica, and Califomia. Appl. Environ. Mierobiol. 39:1085-95.
- ↑ 5.0 5.1 McManus, GB, Fuhrman, JA. 1988. Control of marine bacterioplankton populations: measurement and significance of grazing. Hydrobiologia. 159:51-62.
- ↑ Bernard, C, Rassoulzadegan, F. 1990. Bacteria or microflagellates as a major food source for marine ciliates: Possible implications for the microzooplankton. Mar. Ecol. Prog. Ser. 64:147-155.
- ↑ Servais, P, Billen, G, Rego, JV. 1985. Rate of bacterial mortality in aquatic environments. Appl. Environ. Microbiol. 49:1448-1454.
- ↑ Bergh Ø, Børsheim KY., Bratbak G, Heldal M, 1989. High abundance of viruses found in aquatic environments. Nature. 340:467-468.
- ↑ Fuhrman, JA. 1999. Marine viruses and their biogeochemical and ecological effects. Nature. 399:541-548.
- ↑ 10.0 10.1 Bratbak G, Heldal, M, Norland, S, Thingstad, TF. 1990. Viruses as partners in spring bloom microbial trophodynamics. Appl. Environ. Microbiol. 56:1400-1405.
- ↑ 11.0 11.1 11.2 11.3 Fuhrman J. A., and R. T. Noble. 1995. Viruses and protists cause similar bacterial mortality in coastal seawater. Limnol. Oceanogr. 40:1236-1242.
- ↑ 12.00 12.01 12.02 12.03 12.04 12.05 12.06 12.07 12.08 12.09 12.10 Brum JR, Morris, JJ, Décima, M, Stukel, MR. 2014. Mortality in the oceans: Causes and consequences. Eco-DAS IX. 1-15.
- ↑ Pernthaler, J, Amann, R. 2005. Fate of heterotrophic microbes in pelagic habitats: focus on populations. Microbiol. Mol. Biol. Rev. 69:440-461. doi: 69/3/440
- ↑ Pierce, R. 1992. Ecology of planktonic ciliates in marine food webs. Rev. Aquat. Sci. 6:139-181.
- ↑ Pace, ML. 1988. Bacterial mortality and the fate of bacterial production. Hydrobiologia. 159:41-49.
- ↑ Landry, M, Hassett, R. 1982. Estimating the grazing impact of marine micro-zooplankton. Mar. Biol. 67:283-288.
- ↑ Newell, S, Sherr, B, Sherr, E, Fallon, R. 1983. Bacterial response to presence of eukaryote inhibitors in water from a coastal marine environment. Mar. Environ. Res. 10:147-157.
- ↑ 18.0 18.1 Servais, P, Billen, G, Rego, JV. 1985. Rate of bacterial mortality in aquatic environments. Appl. Environ. Microbiol. 49:1448-1454.
- ↑ 19.0 19.1 Fuhrman, J, Noble, R. 2000. Causative agents of bacterial mortality and the consequences to marine food webs, p. 145-152. In Anonymous Microbial Biosystems: New Frontiers. Proceedings of the 8th International Symposium on Microbial Ecology.
- ↑ 20.0 20.1 Fuhrman, J. 2000. Impact of viruses on bacterial processes, p. 327- 350. In D. L. Kirchman [ed.], Microbial ecology of the oceans. Wiley-Liss.
- ↑ 21.0 21.1 Proctor, LM, Fuhrman JA. 1992. Mortality of marine bacteria in response to enrichments of the virus size fraction from seawater. Mar. Ecol. Prog. Ser. 87: 283-293.
- ↑ Proctor, LM, Azam, F. 1982. Thymidine incorporation as a measure of heterotrophic bacterioplankton production in marine surface waters: Evaluation and field results. Mar. Biol. 66: 109-120.
- ↑ Chin-Leo, G, Kirchman, DL. 1988. Estimating bacterial production in marine waters from the simultaneous incorporation of thymidine and leucine. Appl. Environ. Microbial. 54: 1934-1939.
- ↑ Kirchman, DL. 1992. Incorporation of thymidine and leucine in the subarctic Pacific: Application to estimating bacterial production. Mar. Ecol. Prog. Ser. 82: 301-309.
- ↑ Landry, MR, Lehner-Fournier, JM, Sundstrom, JA, Fagerness, VL, Selph, KE. 1991. Discrimination between living and heat-killed prey by a marine zooflagellate, Paraphysomonas vestita (Stokes). J. Exp. Mar. Biol. Ecol. 146:139-151.
- ↑ Gonzalez, JM, Sherr, EB, Sherr, BF. 1993. Differential feeding by marine flagellates on growing versus starving, and on motile versus nonmotile, bacterial prey. Mar. Ecol. Prog. Ser. 102: 257-267.
- ↑ Proctor, LM, Fuhrman JA. 1990. Viral mortality of marine bacteria and cyanobacteria. Nature 343: 60-62.
- ↑ Landry, M, Hassett, R. 1982. Estimating the grazing impact of marine micro-zooplankton. Mar. Biol. 67:283-288.
- ↑ Pernthaler, J, Amann, R. 2005. Fate of heterotrophic microbes in pelagic habitats: focus on populations. Microbiol. Mol. Biol. Rev. 69:440-461. doi: 69/3/440
- ↑ Wilhelm, SW & Suttle CA. 1999. Viruses and nutrient cycles in the sea viruses play critical roles in the structure and function of aquatic food webs. Bioscience. 49(10):781-788.
- ↑ Gobler, CJ, Hutchins, DA, Fisher, NS, Cosper, EM, Sanudo-Wilhelmy, SA. 1997. Release and bioavailability of C, N, P, Se, and Fe following viral lysis of a marine chrysophyte. Limnol. Oceanogr. 42:1492-1504.
- ↑ Poorvin, L, Rinta-Kanto, JM, Hutchins, DA, Wilhelm, SW. 2004. Viral release of iron and its bioavailability to marine plankton. Limnol. Oceanogr. 49:1734-1741.
- ↑ Steward GF, Smith DC, Azam F. 1996. Abundance and production of bacteria and viruses in the Bering and Chukchi Sea. Mar Ecol Prog Ser. 131:287-300
- ↑ Boras, JA, Sala, MM, Vázquez‐Domínguez, E, Weinbauer, MG, Vaqué, D. 2009. Annual changes of bacterial mortality due to viruses and protists in an oligotrophic coastal environment (NW Mediterranean). Environ. Microbiol.11:1181-1193
- ↑ Almeida, M, Cunha, MA, Alcantara, F. 2001. Loss of estuarine bacteria by viral infection and predation in microcosm conditions. Microb. Ecol. 42:562-571.
- ↑ Guixa-Boixereu, N, Lysnes, K, Pedros-Alio, C. 1999. Viral lysis and bacterivory during a phytoplankton bloom in a coastal water microcosm. Appl. Environ. Microbiol. 65:1949-1958.
- ↑ Bettarel, Y, Sime-Ngando, T, Amblard, C, Dolan, J. 2004. Viral activity in two contrasting lake ecosystems. Appl. Environ. Microbiol. 70:2941-2951.
- ↑ 38.0 38.1 Weinbauer, MG, Hofle, MG. 1998. Significance of viral lysis and flagellate grazing as factors controlling bacterioplankton production in a eutrophic lake. Appl. Environ. Microbiol. 64:431-438.
- ↑ Wells, LE, Deming, JW. 2006. Significance of bacterivory and viral lysis in bottom waters of Franklin Bay, Canadian Arctic, during winter. Aquat. Microb. Ecol. 43:209-221.
- ↑ 40.0 40.1 Miki, T, Jacquet, S. 2008. Complex interactions in the microbial world: under-explored key links between viruses, bacteria and protozoan grazers in aquatic environments. Aquat. Microb. Ecol. 51:195.
- ↑ Middelboe, M. 2000. Bacterial growth rate and marine virus–host dynamics. Microb. Ecol. 40:114-124.
- ↑ Simek, K, Pernthaler, J, Weinbauer, MG, Hornak, K, Dolan, JR, Nedoma, J, Masin, M, Amann, R. 2001. Changes in bacterial community composition and dynamics and viral mortality rates associated with enhanced flagellate grazing in a mesoeutrophic reservoir. Appl. Environ. Microbiol. 67:2723-2733. doi: 10.1128/AEM.67.6.2723-2733.2001
Annotated Bibliography
Almeida, M, Cunha, MA, Alcantara, F. 2001. Loss of estuarine bacteria by viral infection and predation in microcosm conditions. Microb. Ecol. 42:562-571.
This paper found that bacterial mortality by predation and viral lysis exhibited a similar trend in the brackish and marine waters of Ria de Averio, a shallow estuarine ecosystem. In both communities, predation was a more prominent cause of bacterial loss than viral infection. However, an increase in viral mortality rate increased bacterial loss.
Bettarel, Y, Amblard, C, Sime-Ngando, T, Carrias, J, Sargos, D, Garabétian, F, Lavandier, P. 2003. Viral lysis, flagellate grazing potential, and bacterial production in Lake Pavin. Microb. Ecol. 45:119-127.
This paper compared the impact of viral lysis and protist grazing rates with losses of heterotrophic bacteria in a 24-hr study. Based on the estimated viral mediation and assumed clearing rates, flagellates had a greater control over bacterial production than viral lysis. Summed together, both factors was able to account for most of the observed bacterial mortality.
Bettarel, Y, Sime-Ngando, T, Amblard, C, Dolan, J. 2004. Viral activity in two contrasting lake ecosystems. Appl. Environ. Microbiol. 70:2941-2951.
This paper compared bacterial mortality by protisan grazing and viral lysis in two lake ecosystems of differing characteristics, one eutrophic, and the other oligotrophic. Compared to bacteriovory, results showed that viruses contributed more to bacterial mortality in less productive systems such as in the anoxic hypolimnion.
Boras, JA, Sala, MM, Vázquez‐Domínguez, E, Weinbauer, MG, Vaqué, D. 2009. Annual changes of bacterial mortality due to viruses and protists in an oligotrophic coastal environment (NW Mediterranean). Environ. Microbiol.11:1181-1193
This paper conducted a similar experiment as Fuhrman and Nobel (1995), but bacterial mortality was measured for a longer duration with less frequent intervals. The authors showed that not only do viruses play an important role in bacterial losses, they were the dominant cause of bacterial mortality over a two-year period in the oligotrophic waters of NW Mediterranean, contributing to 37% more in bacterial losses than protists.
Bratbak, G, Heldal, M, Norland, S, Thingstad, TF. 1990. Viruses as partners in spring bloom microbial trophodynamics. Appl. Environ. Microbiol. 56:1400-1405.
This paper demonstrated that viruses are a dynamic population that interacts with the bacterial community. Results showed that the collapse of a diatom spring bloom in western Norway was partially attributed to 23% of total viruses attached to dead or senescent diatoms. The authors proposed a “short circuit” loop in which viral lysis recycles organic material back to bacteria, thereby decreasing its availability to higher trophic levels.
Guixa-Boixereu, N, Lysnes, K, Pedros-Alio, C. 1999. Viral lysis and bacterivory during a phytoplankton bloom in a coastal water microcosm. Appl. Environ. Microbiol. 65:1949-1958.
The purpose of this paper’s study was to assess the relative contribution of flagellate grazing and viral lysis in bacterial mortality over the course of a phytoplankton bloom. Experimental evidence showed that bacteriovory was a higher source of mortality than viral lysis in the earlier intervals, and vice versa in the later intervals.
Guixa-Boixereu, N, Vaque, D, Gasol, JM, Pedros-Alio, C. 1999. Distribution of viruses and their potential effect on bacterioplankton in an oligotrophic marine system. Aquat. Microb. Ecol. 19:205-213.
Using a similar approach as Fuhrman and Nobel (1995) in converting percentage of infected cells to bacterial mortality, this paper estimated that with maximal infection values, viruses would only be responsible for 11.1-21.5% of whole bacterial mortality in the oligotrophic NW Mediterranean, though only 2 out of 8 samples were accounted for in the estimation.
Middelboe, M. 2000. Bacterial growth rate and marine virus–host dynamics. Microb. Ecol. 40:114-124.
This paper focused on a single virus-host system as opposed to an entire bacterial community. Experimental evidence showed that the rate of cell lysis and viral production were positively correlated with the bacterial growth rate. Moreover, in studying the dynamics of resistant and sensitive bacteria, the viral lysis rate of sensitive bacteria correlated to an increase in the growth rate of resistant bacteria.
Servais, P, Billen, G, Rego, JV. 1985. Rate of bacterial mortality in aquatic environments. Appl. Environ. Microbiol.49:1448-1454.
This paper discussed an earlier idea that bacterial mortality rates are a result of zooplankton grazing only, with the residual mortality rate being attributed to unknown mechanisms. The authors suggested these mechanisms to include viral-induced lysis or spontaneous auto-lysis, but they have not been tested at the time of publication.
Simek, K, Pernthaler, J, Weinbauer, MG, Hornak, K, Dolan, JR, Nedoma, J, Masin, M, Amann, R. 2001. Changes in bacterial community composition and dynamics and viral mortality rates associated with enhanced flagellate grazing in a mesoeutrophic reservoir. Appl. Environ. Microbiol. 67:2723-2733. doi: 10.1128/AEM.67.6.2723-2733.2001
This paper studied the changes in bacterial community composition in conjunction with mortality rates from protist grazing and viral lysis. While protist grazing and viral mortality contributed equally to bacterial mortality in situ, the grazing-enhanced and grazer-free treatments showed that viral concentrations and frequencies of infected cells was strongly associated with grazing rates. Thus, this paper suggests that protistan grazing may stimulate viral activity.
Steward GF, Smith DC, Azam F. 1996. Abundance and production of bacteria and viruses in the Bering and Chukchi Sea. Mar Ecol Prog Ser. 131:287-300
The authors in this paper studied the impact of viruses and protists on bacterial losses in various locations and depths in the Bering and Chukchi Seas. Using FIB for viruses, and abundance and clearance for protists, they found that both had a comparable contribution to bacterial mortality.
Weinbauer, MG, Hofle, MG. 1998. Significance of viral lysis and flagellate grazing as factors controlling bacterioplankton production in a eutrophic lake. Appl. Environ. Microbiol. 64:431-438.
In this paper, the authors found that grazing was the dominant cause of bacterial mortality in the oxic epilimnion, while viral lysis was more prominent in the anoxic hypolimnion. The grazing rates decreased with depth and oxygen concentration, whereas viral lysis became predominant.
Wells, LE, Deming, JW. 2006. Significance of bacterivory and viral lysis in bottom waters of Franklin Bay, Canadian Arctic, during winter. Aquat. Microb. Ecol. 43:209-221.
This paper studied the importance of grazers and viruses in bacterial mortality in the bottom waters of the winter Arctic. While grazing rates were detectable due to low concentrations of bacteria and flagellates, bacterial mortality was primarily attributable to viruses. As a result of increased cycling of bacteria-derived organic material, this helps bacteria sustain through the winter, but prevents transfer to higher trophic levels.